Artemether (INN) is an antimalarial for the treatment of multi-drug resistant strains offalciparum malaria. It's combination (co-formulation) with Lumefantrine has first been marketed by Novartis under the brand names Riamet and Coartem.
Today, this combination therapy is available as generic from several manufacturers.
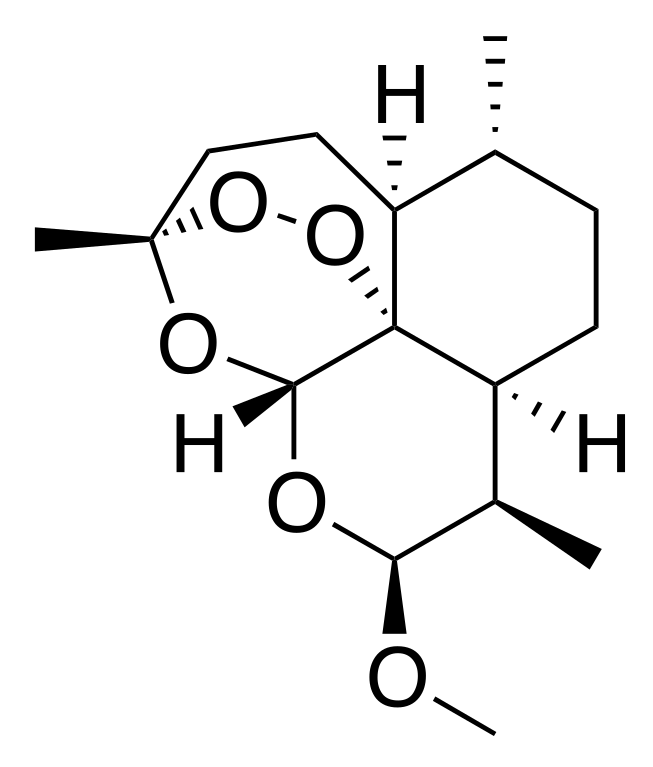
It is a methyl ether derivative of artemisinin, which is a peroxide lactone isolated from theChinese antimalarial plant, Artemisia annua. It is also known as dihydroartemisinin methyl ether, but its correct chemical nomenclature is (+)-(3-alpha,5a-beta,6-beta,8a-beta, 9-alpha,12-beta,12aR)-decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin. It is a relatively lipophilic and unstable drug.
Artemether is highly effective against the blood schizonts of both malarial parasites P. falciparum and P. vivax. It is available as mono-therapy but usually applied in combination with lumefantrine in clinical treatments of malaria. World Health Organization guidelines for the treatment of uncomplicated falciparum malaria recommend the use of this artemisinin-based combination therapy, and approved by Swissmedic in December 2008 and recently approved by the United States Food and Drug Administration. Zambia was the first African country to adopt artemether/lumefantrine (commonly called Coartem) as first-line therapy in national malaria treatment guidelines in 2002. Clinical records show that by 2008, the rates of in-patient malaria cases and deaths decreased by 61% and 66%, respectively, compared with the 2001-2002 reference period. In South Africa also the number of malaria-related outpatient cases and hospital admissions to each fall by 99% from 2001 to 2003, and malaria-related deaths decreased by 97% over the same period.[2] The efficacy of the six-dose regimen of Coartem has been confirmed in many different patient populations around the world, consistently achieving 28-day polymerase chain reaction-corrected cure rates of >95% in the evaluable population, rapidly clearing parasitaemia and fever, and demonstrating a significant gametocidal effect, even in areas of widespread parasite resistance to other antimalarials. Coartem is much more effective than quinine, the classical antimalarial. Randomised clinical trial in Uganda shows cure rate of malaria as high as 96% in the Coartem-treated group compared with 64% for the quinine group. For Plasmodium vivax infection, combination withpiperaquine is more effective than Coartem.

PLANT
Artemether has been assigned to category C by the FDA on the basis of animal data which shows an association with fetal-loss and deformity. However, clinical data appears to show that artemether is safe in pregnancy. A meta-analysis of 14 clinical trials that looked at artemether use in a total of 945 pregnant women did not find evidence of harm, and a clinical trial of artemether-lumefantrine designed to look at this question found fewer adverse events in the 138 pregnant women treated with artemther-lumefantrine than women treated with quinine.

No comments:
Post a Comment