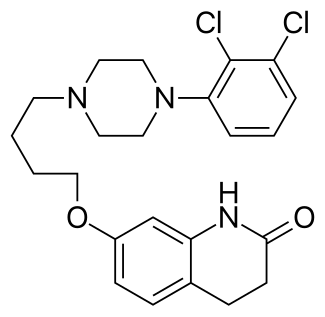
Aripiprazole (brand names: Abilify, Aripiprex, OPC-14597,) is an atypical antipsychotic drug with the chemical name 7-[4-(2,3-dichlorophenyl)-1-piperazinyl]-butyloxy]-3,4-dihydro-2(1H)-quinolinone. It was first discovered by the Otsuka Pharmaceutical Company, Ltd., based in Japan, and in collaboration with Bristol-Myers Squibb began to market the drug in the United States following the approval by the Food and Drug Administration for schizophrenia in November 2002. According to IMS Health, Abilify was the fourth top-selling drug in the United States in 2011, with sales of $5.2 billion.
Chemical Structure of Aripiprazole (brand names: Abilify)
Chemical Synthesis of Aripiprazole(active ingredient for Abilify)
Experimental Procedures for the preparation of Aripiprazole (Abilify)
US 5,006,528 discloses process for the preparation of Aripiprazole in two steps. The first step comprises synthesis of 7-(4-bromobutoxy)-3,4-dihydrocarbostyril (7-BBQ) by alkylating the hydroxy group of 7-hydroxy-3,4-dihydrocarbostyril (7-HQ) with 1 ,4-dibromobutane using potassium carbonate in water at reflux temperature for 3 hours to obtain 7-BBQ in 68% yield. The resulting 7-BBQ is further reacted with 1- (2,3-dichlorophenyl)-piperazine to obtain Aripiprazole.
Preparation of 7-(4-bromobutoxy)-3,4-dihydro-2(1H)quinolinone (7-(4-bromobutoxy)-3,4-dihydrocarbostyril; 7-BBQ)
7-Hydroxy-3,4-dihydro-2(1H)-quinolinone (aka 7-Hydroxy-3,4-dihydrocarbostyril, 60gm) and potassium carbonate (76.3 gm) were taken in acetonitrile (1200ml) at room temperature. To this tetra butyl ammonium iodide (13.7 gm) and 1 ,4-dibromobutane (238.5gm) were added and heated at 40- 45°C for 24 hours. Reaction mass was cooled upto room temperature and was filtered off. The resulting filtrate was distilled off under vacuum. The resultant mass was cooled to 25-30°C and cyclohexane (300 ml) was added under stirring. The resulting solid was filtered off and was dried. The resulting solid was taken in water and was stirred for few minutes. The solid was filtered and dried under vacuum at 55-60°C for 20 hours to obtain title compound. mp 110.5-111 °C; 1H NMR (DMSO-d6) ä 1.81 (2H, m, -CH2-), 1.95 (2H, m, -CH2-), 2.41 (2H, t, J ) 7 Hz, -CH2CO-), 2.78 (2H, t, J) 7 Hz, -CH2-C-CO-), 3.60 (2H, t, J ) 6 Hz, -CH2Br), 3.93 (2H,t, J ) 6 Hz, O-CH2-), 6.43 (1H, d, J ) 2.5 Hz), 6.49 (1H, dd, J) 2.5, 8 Hz), 7.04 (1H, d, J ) 8 Hz), 9.98 (1H, s, NHCO). Anal. (C13H16NO2Br) C, H, N.
Yield: 73-75%; Purity: 93-95%
Preparation of Aripiprazole (7-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one)
7-(4-Bromobutoxy)-l ,2,3,4-tetrahydroquinolin-2-one (50 gm) was taken in acetonitrile (500 ml) at 25-30°C. To this potassium carbonate (67.2 gm) and l-(2,3- dichlorophenyl) piperazine hydrochloride (44.9gm) were added under stirring. The reaction mixture was refluxed at 80-85°C for 8 hours. The reaction mass was cooled to room temperature, filtered and the resulting solid was washed with acetonitrile. To the resulting solid, water was added and was stirred. The solid was filtered off, washed with water and dried under vacuum at 75-80°C for 15 hrs. The resulting crude aripiprazole was crystallized from isopropyl alcohol and water to obtain title compound. Yield: 75-80%; Dimer Impurity: <0.1%. 1H NMR: DMSO-d6 d 9.96 [1H, s, NH]; 7.29 [2H, m, Ar]; 7.13 [1H, q, Ar]; 7.04 [1H, d, Ar]; 6.49 [1H, dd, Ar]; 6.45 [1H, d, Ar]; 3.92 [2H, t, -CH2-O-]; 2.97 [4H, bb,2(-CH2-)]; 2.78 [2H, t, -CH2-N2-)]; 2.39 [4H, m, 2(-CH2-)]; 1.73 [2H, m, - CH2-]; 1.58 [2H, m, -CH2-]. IR:cm-1 3193; 2939; 2804; 1680; 1627; 1579; 1520; 1449; 1375; 1270; 1245; 1192; 1169; 1045; 965; 649; 869; 780; 712; 588.
Preparation of aripiprazole anhydrous Type I using isopropyl alcohol and water
Crude aripiprazole (30 g) was taken in isopropyl alcohol (600 ml) and was heated to 80-85°C. Water (90 ml) was added at the same temperature. Activated carbon was added and the mixture was stirred for 30 minutes at the same temperature. The resulting hot solution was filtered and the bed was washed with hot isopropyl alcohol. The resulting filtrate was cooled to 25-30°C for 4 hours. The resulting solid was filtered, washed with isopropyl alcohol and dried under suction for 1 hour. The resulting wet solid was dried in preheated oven maintained at 100-105°C for 6 hours to obtain title compound.
Yield: 87-89% HPLC Purity: 99.89
Anhydrous crystal D: Below detectable limit (BDL) at limit of detection 1%.
Hydrate A: Below detectable limit (BDL) at limit of detection 1 %.
Particle size distribution: d10=15.83 m, d50=60.12 m, d90=144.99 m
Preparation of aripiprazole anhydrous Type I using ethanol and water
Crude aripiprazole (15 g) was taken in ethanol (300 ml) and water (45 ml) and was heated to 80-85°C for 1-2 hours. The resulting mixture was cooled to 25-30°C within 4 hours and stirred for 3 hours. The resulting solid was filtered and dried under suction for 1 hour. The resulting wet solid was dried in preheated oven maintained at 100-105°C for 3 hours to obtain title compound. Yield: 90% HPLC Purity: 99.9%
Anhydrous crystal D: Below detectable limit (BDL) at limit of detection 1%.
Hydrate A: Below detectable limit (BDL) at limit of detection 1%.
Particle size distribution: d10=22.01 m, d50=105.10 m, d90=232.97 m
References For the Process of Aripiprazole (Abilify,)
Yasuo Oshiro, Seiji Sato, Nobuyuki Kurahashi, Tatsuyoshi Tanaka, Tetsuro Kikuchi, Katsura Tottori, Yasufumi Uwahodo, and Takao Nishi; Novel Antipsychotic Agents with Dopamine Autoreceptor Agonist Properties: Synthesis and Pharmacology of 7-[4-(4-Phenyl-1-piperazinyl)butoxy]- 3,4-dihydro-2(1H)-quinolinone Derivatives; J. Med. Chem. 1998, 41, 658-667
Yasuo Oshiro, Seiji Sato, Nobuyuki Kurahashi; Carbostyril derivatives, Otsuka Pharmaceutical Co., Ltd; US patent 5006528;Issue date: Apr 9, 1991
BANDO, Takuji, YANO, Katsuhiko, FUKANA, Makoto, AOKI, Satoshi ;Method for producing fine particles of aripiprazole anhydride crystals b; OTSUKA PHARMACEUTICAL CO., LTD., WO 2013002420 A1
ZHENG Siji, LIU Xiaoyi, FU Linyong, TAN Bo, ZHOU Min: ARIPIPRAZOLE MEDICAMENT FORMULATION AND PREPARATION METHOD THEREFOR. / FORMULATION DE MÉDICAMENT ARIPIPRAZOLE ET SON PROCÉDÉ DE PRÉPARATION. / SHANGHAI ZHONGXI PHARMACEUTICAL January 2013: WO 2013/000391
CN 201210235157
CN102846543A
CN102850268A;
CN101781246A
GUPTA, Vijay Shankar, KUMAR, Pramod, VIR, Dharam ;Process for producing aripiprazole in anhydrous type i crystals;JUBILANT LIFE SCIENCES LIMITED;WO 2012131451 A1
SRIVASTAVA JAYANT GUPTA Vijay Shankar;Improved process for the preparation of 7.(4-bromobutoxy) 3,4-dihydrocarbostyril, a precursor of aripiprazole;wo2011030213 A1
No Generic Abilify in the US until April 2015
On May 7, 2012, The U.S. Court of Appeals for the Federal Circuit ruled in favor of Otsuka Pharmaceutical Co., Ltd. in its patent litigation against several companies including Israel-based Teva and Weston, Ontario-based Apotex seeking FDA approval to market generic copies of Abilify®. (
see the pdf file for the decision upholding the Otsuka patent here). The Federal Circuit affirmed a decision of the U.S. District Court for the District of New Jersey holding that the asserted claims of
U.S. Patent No. 5,006,528 (
pdf file here) covering aripiprazole, the active ingredient in Abilify®, are valid, thus maintaining patent and regulatory protection for Abilify® in the U.S. until at least
April 20, 2015. The case is Otsuka Pharma Co. v. Sandoz Inc., 2011-1126 and 2011-1127, U.S. Court of Appeals for the Federal Circuit (Washington). The lower court case is Otsuka Pharmaceutical Co. v. Sandoz Inc., 07cv1000, U.S. District Court for the District of New Jersey (Trenton).

Chemical Name for Aripiprazole(active ingredient for Abilify): 7-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one
CAS number 129722-12-9
Aripiprazole can be synthesized beginning with a dichloroaniline and bis(2-chloroethyl)amine:
- U.S. Patent 5,006,528